Quality control is required throughout the entire development process, from drug substance and raw material control, through intermediate drug product testing, to finished drug product release testing, bioavailability and stability studies. By having a comprehensive understanding of the physical and chemical properties of your drug substances and drug products, you can develop the most appropriate drug product formulations. Nikyang represents the pharmaceutical testing leader, SOTAX in providing the testing equipment in the development and production processes for our customers in the pharmaceutical industry. Our extensive market and process know-how enable us to meet our customers’ requirements and comply with the USP, EP and CP requirements relevant to them. We are also open to cooperation with new industries.
Dissolution

AT
A fully scalable and modular dissolution sampling apparatus

AT 70smart
Fully automatic Dissolution Tester

CE 7smart
Flow through cell Dissolution Tester

DVC-24
Dissolution Vessel Cleaner
Physical Testing

DT50
Automatic Tablet Disintegration Tester

DT2
Manual Disintegration Tester

FT2
Friability Tester

PF1
Powder and Granulate Flowability Tester

ST50
Semi-automatic Tablet Hardness Tester

MT50
Manual Tablet Hardness Tester

AT50
Fully Automatic Tablet Hardness Tester

TD1
Tap Density Tester
Sample Preparation

TPW
Automated Tablet Processing Workstation
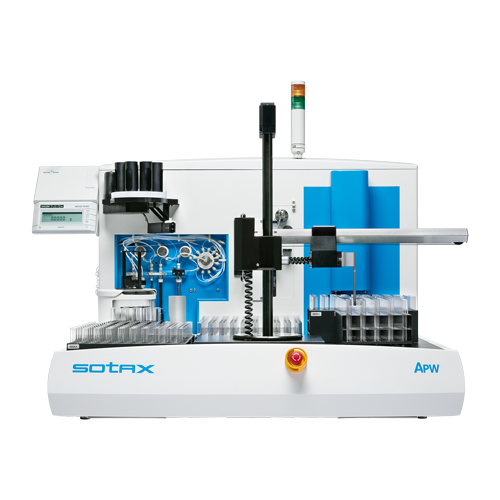
APW
API Workstation

MPS
Media Preparation Station for dissolution media
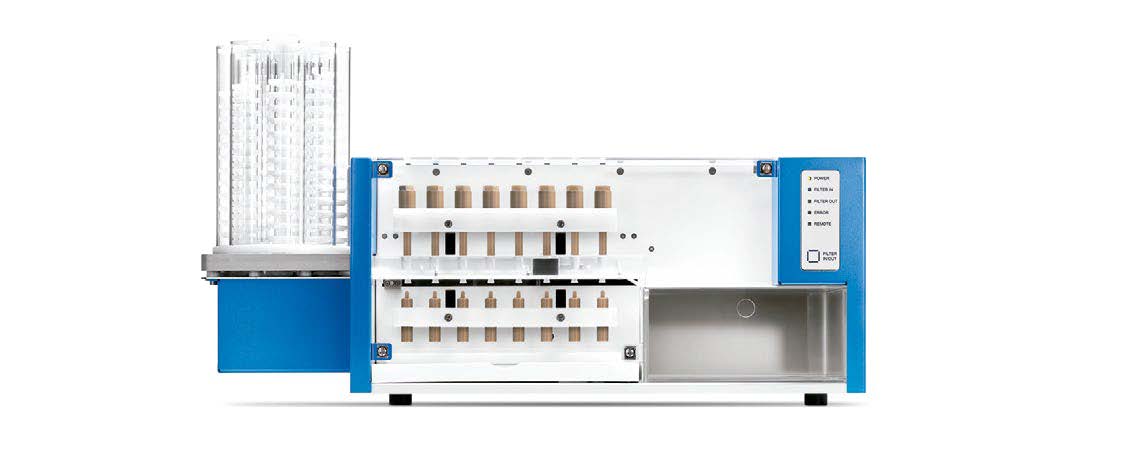
FS
Automated filter change workstation